
Studying the Functions of Chromatin Modifications Using 'Designer' Chromatin
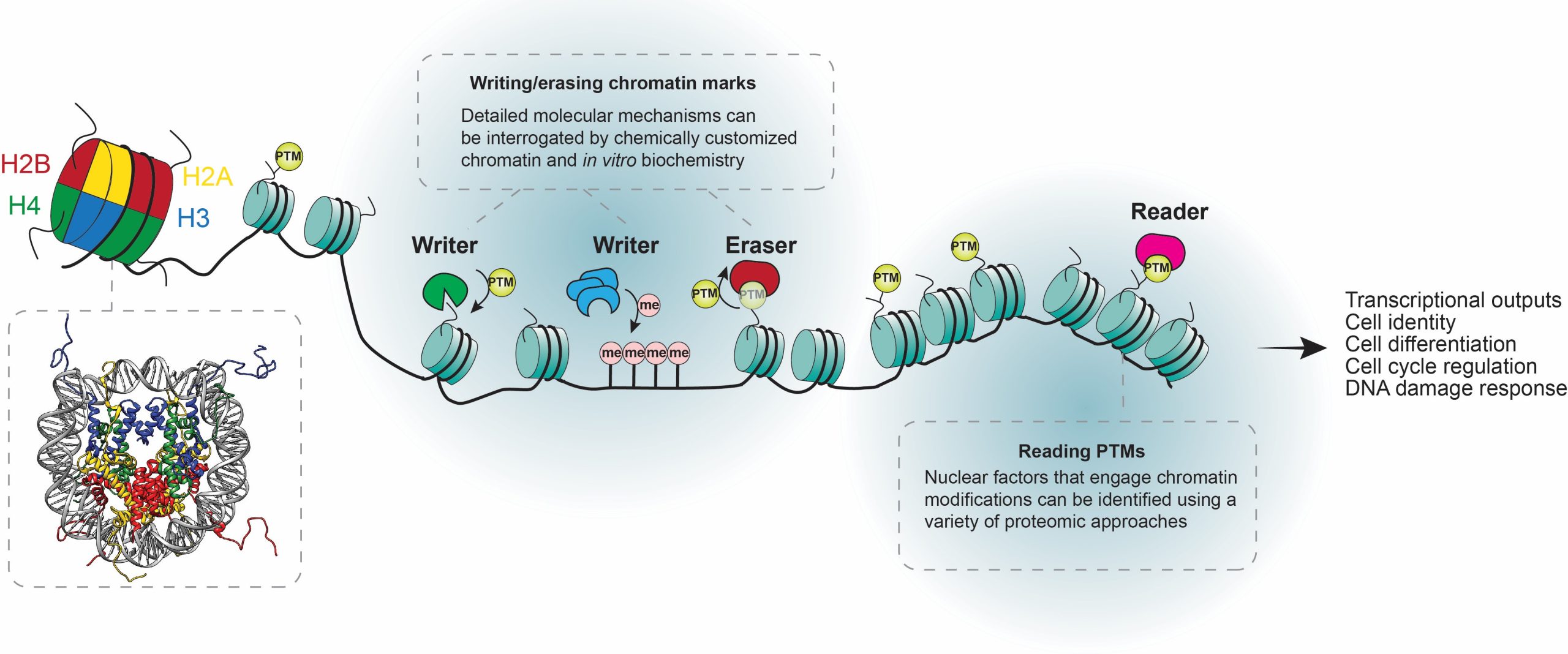
Chromatin is the complex of DNA and proteins that packages our genetic material inside cells, playing a key role in controlling which genes are active or silent. At the heart of this regulation are histones—proteins around which DNA wraps like thread on a spool. These histones can be chemically modified through post-translational modifications (PTMs), such as adding small groups like acetyl or methyl, which act like switches or signals to influence gene expression and other DNA-templated processes.
In our lab, we tackle open questions about how these PTMs function and are maintained over time: Are certain modifications sufficient on their own to drive gene activity? How do they interact with other cellular factors? And how are they preserved or diluted during cell division? To answer these, we use ‘designer’ chromatin—chemically synthesized chromatin with precise, custom PTMs—that we introduce into living cells. This controlled approach allows us to observe direct effects on gene expression and epigenetic inheritance, harnessing chemistry to uncover fundamental mechanisms in chromatin biology, with potential implications for health and disease.
Photocatalysis in living cells for studying protein-protein interactions
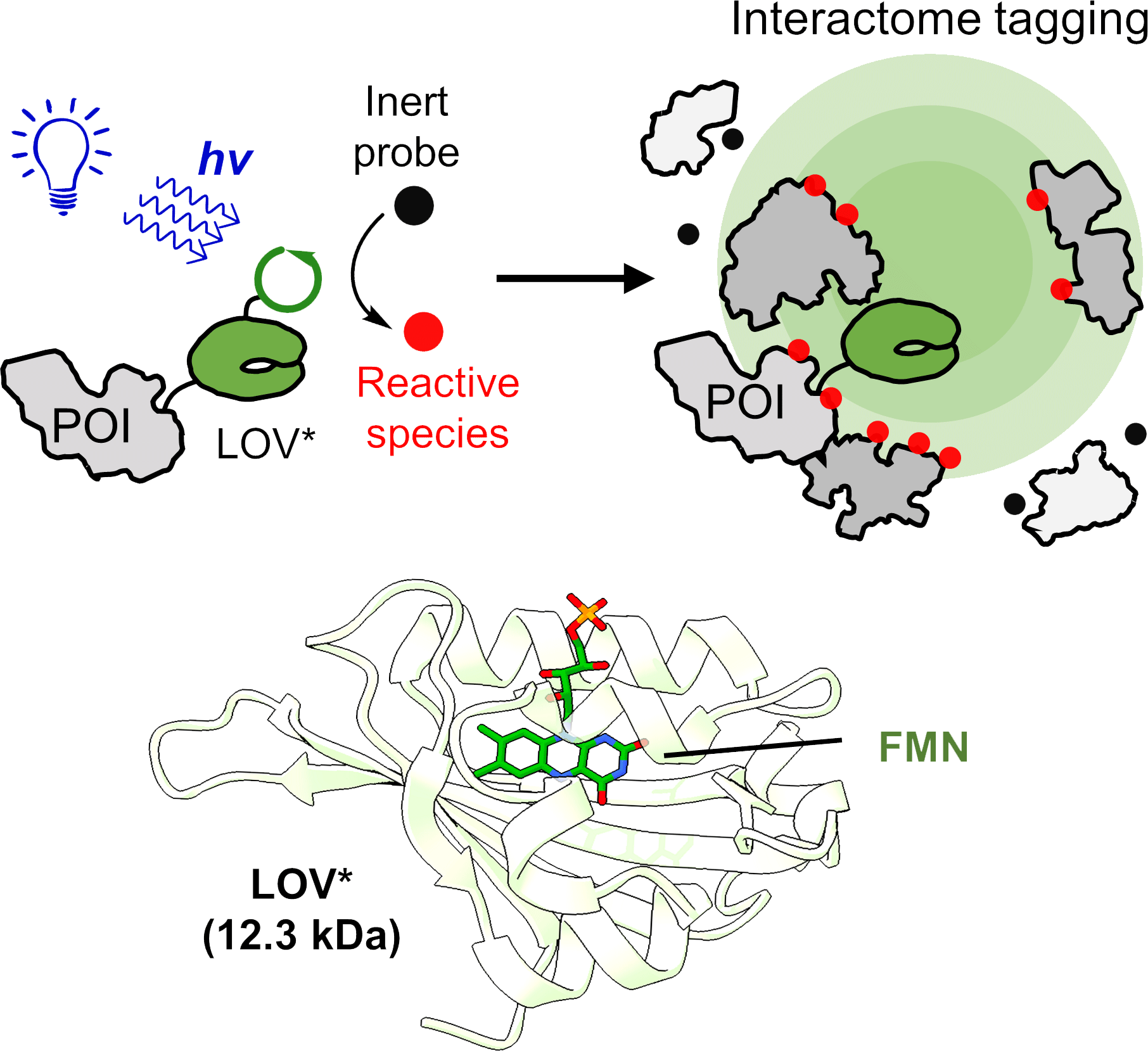
Visible light-mediated photocatalysis is a powerful strategy in organic chemistry, enabling novel synthetic transformations. As photocatalysis is often performed under mild conditions and is compatible with water, it holds great potential for chemically manipulating biological systems. Our lab aims to develop new technologies that will enable photocatalysis inside living cells. Such chemical tools will provide invaluable information about Biology.
Photocatalysis requires a catalyst, usually a metal complex or an organic small-molecule dye. Such a catalyst does not naturally exist inside cells and needs to be provided exogenously, a demand that traditionally limits the applicability of photochemistry in vivo.
We asked ourselves – Can we use proteins, which are genetically encoded, as endogenous photocatalyst? The answer is yes!
We recently developed LITag (https://www.pnas.org/doi/10.1073/pnas.2219339120), which relies on genetically encoded photocatalysts to achieve photo-proximity labeling. This method is used to study protein-protein interactions in living cells. We continue to design photo-proximity labeling tools that will deepen our understanding of protein-protein interaction regulation in various dynamic biological processes. In addition, we use our unique in vivo photochemistry approach to accomplish new chemical transformations in living cells.